10F, Building B, Erqi Center, Erqi District,
Zhengzhou City,
Henan Province, China
Wit:+86 15138685087
(WhatsApp/Wechat)

10F, Building B, Erqi Center, Erqi District,
Zhengzhou City,
Henan Province, China
Wit:+86 15138685087
(WhatsApp/Wechat)
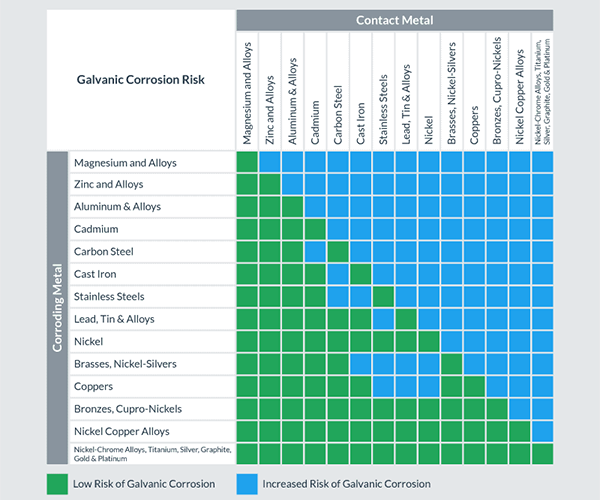
We can place galvanized steel and copper together under normal circumstances, but galvanic corrosion may occur under certain conditions (such as when there is an electrolyte). So why do the two corrode? Under what circumstances does corrosion occur? What protective measures can we take to prevent electrolysis? Can we also galvanize copper? The following contents will answer your questions one by one.

zinc and copper

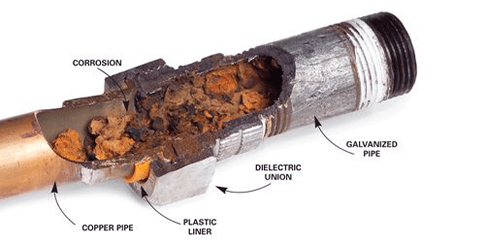
copper and gi steel pipe
When we consider the combination of copper and galvanized steel, there are several key factors to focus on:
1. Galvanized steel is an alloy material that covers the surface of ordinary steel with a layer of zinc. This zinc layer is mainly used to enhance the corrosion resistance of steel.
2. Copper is a metal with good electrical and thermal conductivity, and also has a certain degree of corrosion resistance.
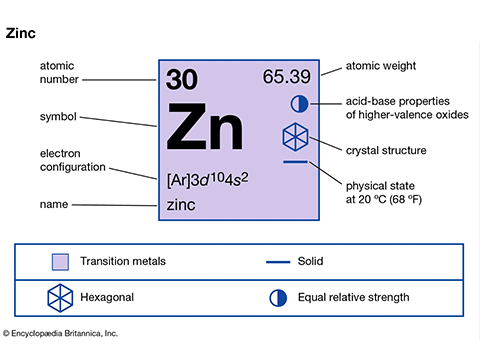
Chemical element zinc
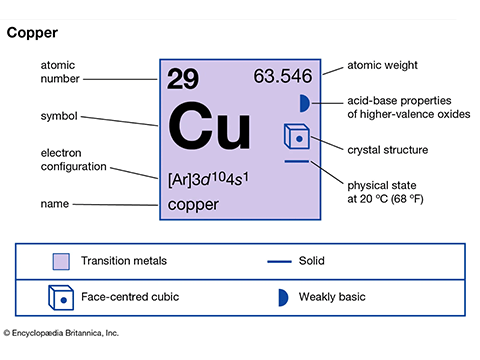
Chemical element copper
1. Generally speaking, galvanized steel and copper are physically compatible and can be used together.
2. However, in certain environments or conditions, such as the presence of electrolytes (moisture, salt), the two may undergo electrochemical corrosion, which is called “galvanic corrosion” or “contact corrosion.”
Galvanized cross section
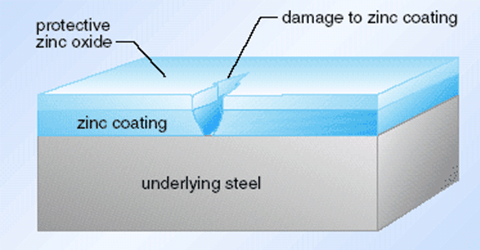
Zinc layer protection diagram
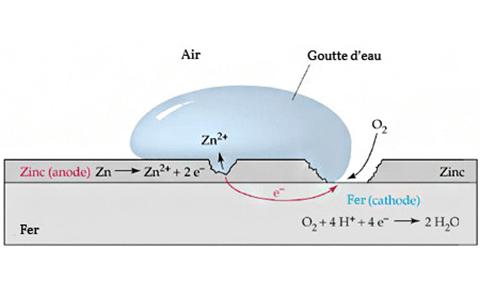
When two dissimilar metals, such as copper and zinc, the outer layer of galvanized steel, come into contact and are exposed to an electrolyte, such as water, one metal will corrode faster than when it is not in contact with the other.
copper and zinc
This is called electrochemical corrosion or bimetallic corrosion. In the case of copper and galvanized steel, the zinc coating on the steel will corrode faster when it comes in contact with the copper, especially in a humid or hot environment.
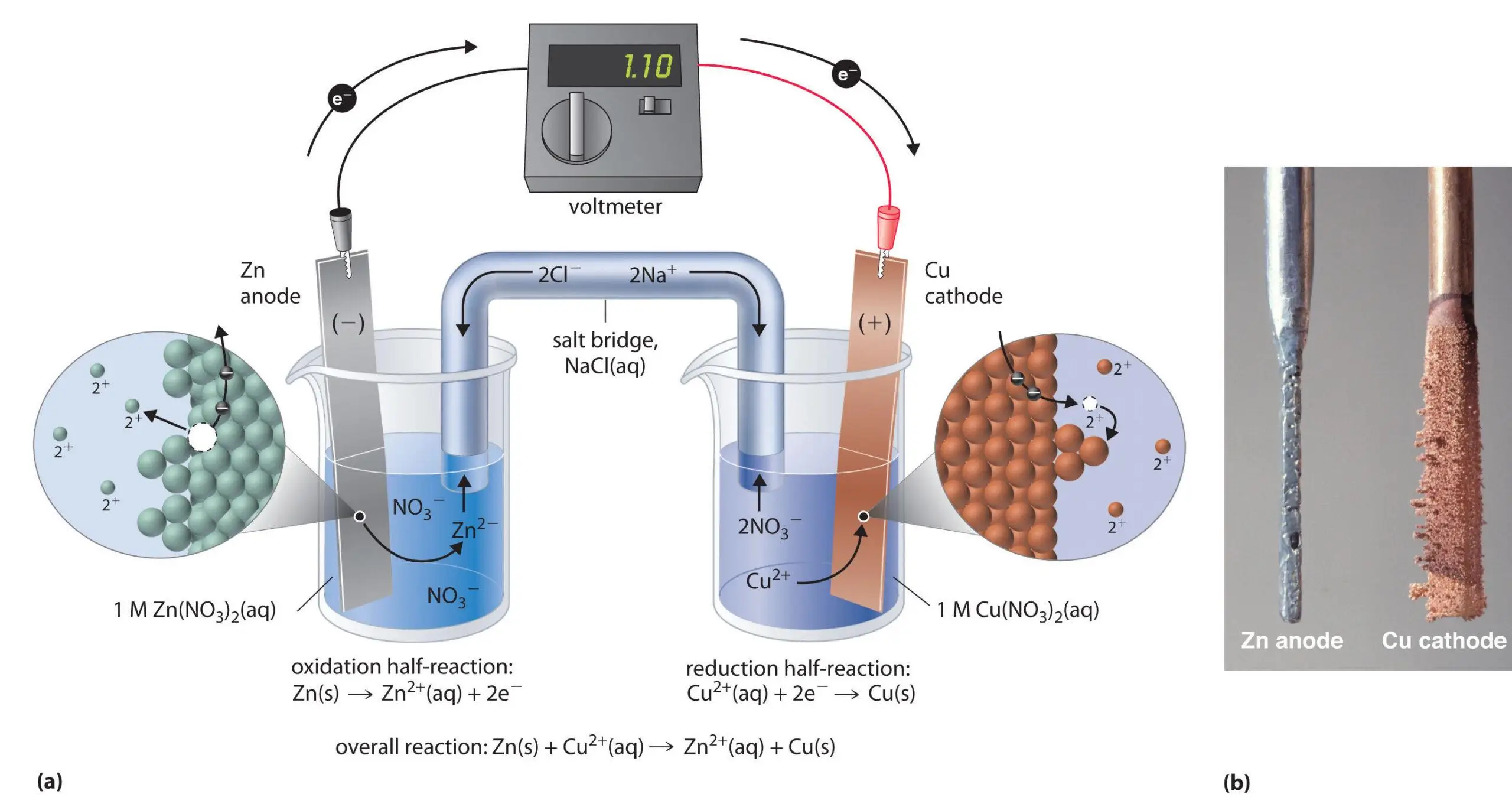
Electrochemical reaction of zinc and copper ions
This is because zinc and copper have different positions in the electrochemical sequence, and when they are in contact with each other and an electrolyte, zinc will act as an anode and corrode faster.
In simple terms, although zinc is active, its ability to resist air + water corrosion in a neutral environment is stronger than that of copper. ZnO is also relatively dense. Zinc does not change much when placed in water, while copper will grow verdigris after a few days, and Zn can be sacrificed to protect Cu.
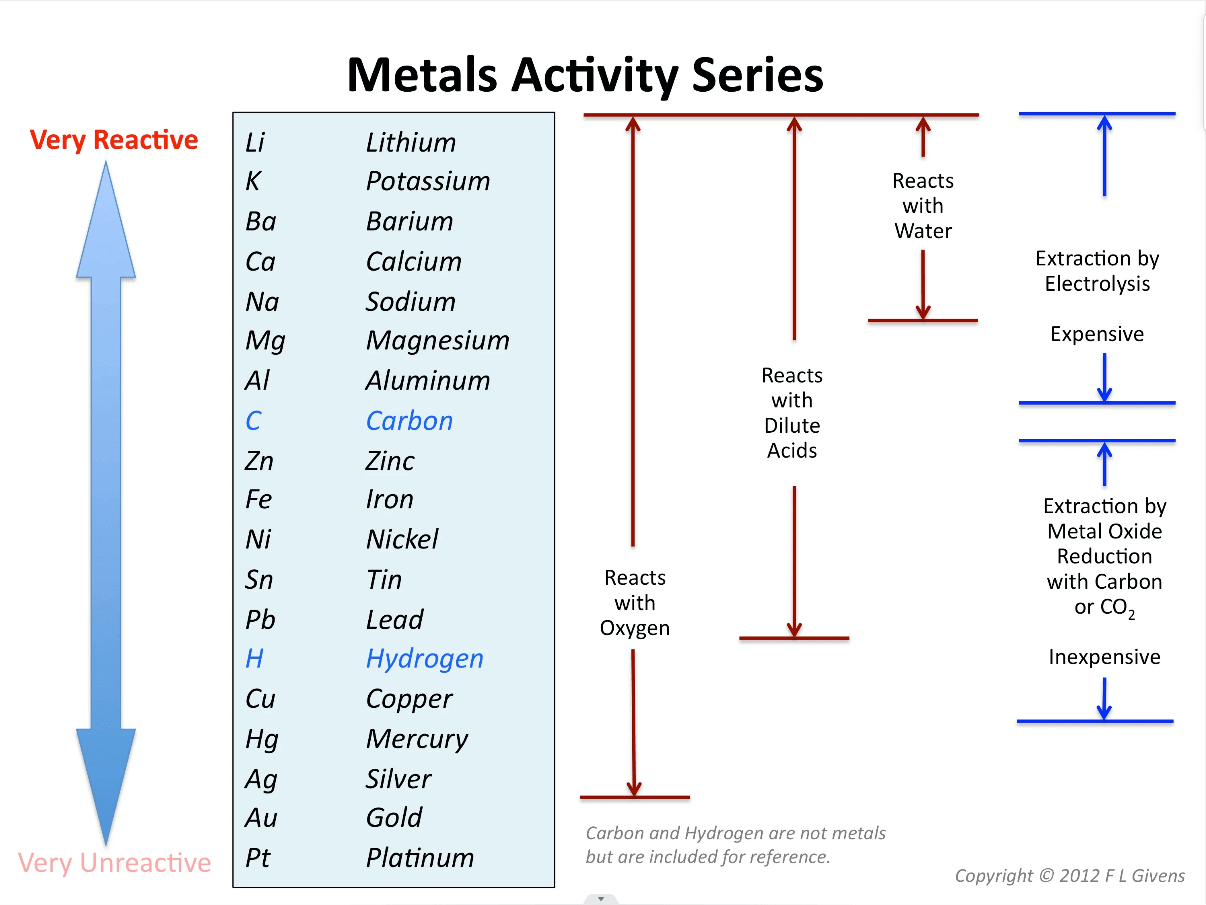
Metal Reactivities Series
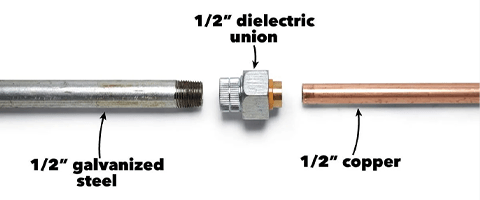
Suppose we need to establish a connection or contact between galvanized steel and copper, and there may be electrolytes in the environment. In that case, it is recommended to take the following measures to reduce or prevent galvanic corrosion:

Coating protection

Protective coating
Bolts with insulating strips
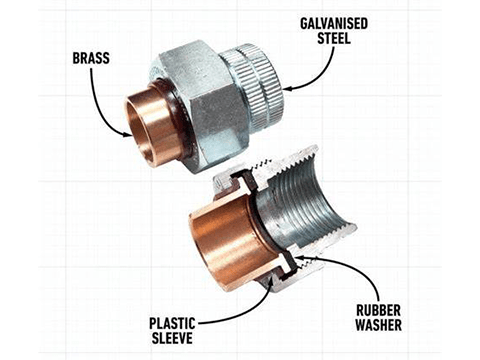
Insulation gasket included
Insulation washers
Insulation material isolation
We can galvanize copper. Although copper itself is not easy to rust, in some specific cases, galvanizing is still chosen to enhance its corrosion resistance and extend its service life.
Zinc has active chemical properties. In the air at room temperature, a thin and dense basic zinc carbonate film is formed on the surface to prevent further oxidation. About half of the world’s total zinc consumption is used for galvanizing.
Galvanizing is a process of coating the surface of iron products that are not easy to rust with a layer of zinc. The main purpose is to prevent it from rusting and improve its corrosion resistance.
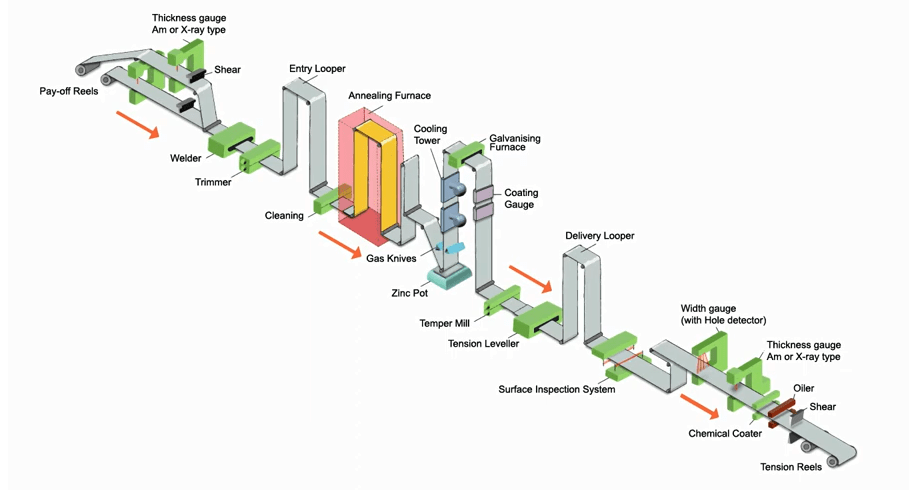
Hot dip galvanizing process of steel coil
1. Copper galvanizing is a process of electrochemically plating a layer of zinc on the copper surface.
The principle is to use copper as the anode and zinc as the cathode. The positive copper is dissolved by an external power supply, and a zinc layer is precipitated on the cathode zinc.
In this process. It is necessary to ensure that the copper surface is clean and remove oxides and impurities. At the same time, the electrolyte is usually a hydrochloric acid solution containing zinc ions.
2. In addition, there is hot-dip galvanizing, which is to put the copper parts into a 500° zinc solution for galvanizing. Continuous hot dipping can make all corners of the copper-plated parts evenly and densely coated with a zinc protective layer.
galvanic corrosion chart
The thickness of the galvanized copper bar should comply with the provisions of the national standard GB/T 5231-2012, and the thickness of the galvanized layer should be between 0.02mm and 0.1mm.
The thickness of the galvanized copper sheet shall comply with the provisions of the national standard GB/T 6461, which specifies the relevant requirements for the thickness of galvanized iron and steel sheets, steel wires, and steel pipes.

under high humidity

high temperature
It can effectively prevent copper from being corroded by the environment and extend its service life.
The galvanized copper can still maintain good conductivity and its electrical properties will not be affected by galvanizing.
Copper-zinc compatible fasteners
It can reduce the problems that occur during the use of copper and improve its durability and reliability.
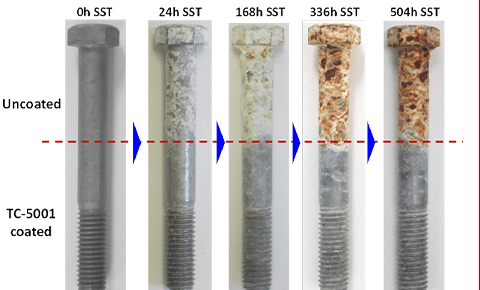
Galvanizing comparison
Zinc layer oxidation
Despite the potential for galvanic corrosion, copper and galvanized steel are still widely used in a variety of applications such as plumbing, wiring, and hardware. It is important to note that, we can minimize galvanic corrosion by taking appropriate protective measures.
Magnetron

basin
construction
In summary, we can put copper and galvanized steel together, but be aware of electrochemical corrosion and take necessary measures to prevent it, especially in humid or hot environments. In addition, we also can galvanize copper, and galvanized copper has many advantages, such as better corrosion resistance, conductivity, and durability.
Wanzhi Steel sells a wide range of durable galvanized steel products, such as galvanized steel coil and color-coated steel coil.


We can offer GI steel in coil, sheet, tube, and strip, with or free of spangles. The available thickness ranges from 0.12 to 4 mm and the width is from 600 to 1,500mm.
Welcome to contact us for more details. The MOQ is 5 tons, and the price is favorable. Go get a free quote!





